Combustion Theory
If you have been following along through the whole training material, you know that a plant can be thought of as an energy conversion factory. The thermodynamics and heat transfer discussed to this point do not address the beginning of the process, the burning of the fuel in the boiler, called combustion. This section describes combustion and factors that affect combustion
Principles of Combustion
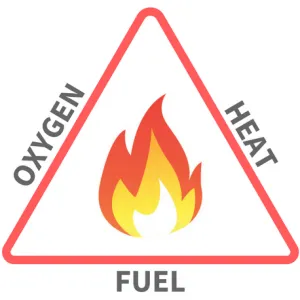
Combustion is defined as the rapid chemical combination of oxygen with the combustible elements of a fuel. This chemical reaction produces heat. The goal of combustion in boilers is to burn the fuel completely to get as much heat as possible from the fuel. The three basic things needed for combustion are shown in the combustion triangle.
Fuel:
Fossil fuels like those burned in a boiler are made up principally of the chemical elements – carbon (C), hydrogen (H), and sulfur (S). These elements combine with oxygen (air) to produce heat. The oxygen (air) must be thoroughly mixed with the fuel to achieve combustion.
Good mixing of the fuel and the combustion air can be enhanced by increasing the ratio of the fuel’s surface area to its weight. The more surface area a given weight of fuel has, the easier it is for the oxygen in the air to mix with the fuel. Several methods are used to increase the surface area of the fuels to make it easier for the fuel to mix with air. Increasing the surface area of coal is the most difficult while natural gas is the easiest.
To burn coal as a fuel it is crushed, ground, or gasified. Oil is sprayed through a nozzle under pressure to produce a fine mist creating a large surface area. Heavy oil (No. 6 oil is very thick at normal temperature) is heated so it can be sprayed under pressure through a nozzle to make a fine mist. Natural gas is mixed with air in a burner or combustor. Because natural gas is already a gas, nothing needs to be done to increase its surface. However, the gas still must be evenly mixed with the air to achieve efficient combustion.
Heat:
Fuels need heat to start the combustion reaction. Heat raises the fuel temperature until the combustion reaction starts. The temperature at which combustion starts is the ignition temperature. The ignition temperature of some substances, including fuels used in boilers, may be relatively high. In modern boiler furnaces, small oil or gas fires usually supply the heat needed to ignite the primary fuel. These small fires, called ignitors, are usually lit by a high-energy electric spark produced by high-voltage transformers. After the ignitor ignites the main fuel, the heat of combustion from the main fuel is sufficient to make the combustion self sustaining, then the ignitor can be put out.
Oxygen
The source of oxygen for combustion in the boiler is air. Air in the atmosphere is a mixture of about 21% oxygen, 78% nitrogen, and 1% other gases. Typically 3 to 4 pounds of oxygen is necessary to burn each pound of fuel. Since air is only 21% of the air supplied, this means that about 15 to 20 pounds of air must be supplied to burn each pound of fuel. The air used to supply oxygen for combustion must be well mixed with the fuel to achieve good combustion. Additionally, good combustion needs enough air to be sure that oxygen contacts the surface of all the fuel.
Combustion products and excess air
When fossil fuels are burned, combustion products as well as heat are produced. The term flue gas is often used to describe the combustion products. Flue gas is actually a mixture of several different gases.
The exact composition of the flue gas depends upon several factors, including the chemical composition of the fuel burned, and whether or not all of the fuel has been burned completely.
When fossil fuels burn completely, the carbon in the fuel is converted to carbon dioxide and the hydrogen is converted to water vapor. Sulfur is converted to oxides of sulfur. All of these gases are present in flue gas, however the majority of the flue gas is nitrogen. This is true because there is so much nitrogen in the combustion air supplied to burn the fuel. The nitrogen does not enter into the combustion reaction (with one notable exception, NOx)
There is another gas found in flue gas, oxygen. To understand why there is oxygen in the flue gas it is necessary to understand the term excess air.
The basis for the term excess air is based upon another concept called the “theoretical air” (also called stoichiometric air). The theoretical air is calculated using chemical combustion calculations. It is the exact amount of air that is required to burn a given amount of fuel (usually one pound) to completion, assuming that there were perfect mixing of the fuel and oxygen in the air. If less than the theoretical air were to be supplied, some of the fuel would not be burned. This would be inefficient because it would mean that part of the heating value of the fuel would be lost.
In reality, however, if only the theoretical air is supplied to a real combustion process, not all of the fuel is burned. This happens because of the difficulty of getting adequate mixing of the fuel and air. It is necessary, therefore, to supply more than the theoretical air to assure complete combustion of the fuel. The amount of air above the theoretical air that must be supplied is called excess air.
Typically, the harder the fuel is to burn, the more excess air is required to get complete combustion. In fossil fuels, coal is the most difficult to burn compared to oil and gas, so coal requires the greatest amount of excess air. Oil requires less excess air than coal and gas still less.
The amount of excess air is determined by the amount of oxygen in the flue gas. If only theoretical air were supplied and all the fuel were to be burned somehow, there would be no oxygen in the flue gas, since it would all have combined with the fuel. Since excess air is supplied in real combustion processes, however, there is a small amount of oxygen in the flue gas, usually about 1% to 4%.
Since the amount of oxygen in flue gas is a good indicator of the combustion air supplied to a combustion process, it is many times used as a way to control the combustion process in boilers. A flue gas analysis instrument continuously measures the oxygen in the flue gas and the output of that instrument is used to control the air supplied to that process. The control is generally done by adjusting dampers at the inlet or discharge of a fan (usually called the forced draft fan) that supplies air to the burner where combustion takes place.
Combustion products of incomplete combustion
Flue gas may contain other constituents if there is incomplete combustion. Incomplete combustion occurs generally because of insufficient excess air and/or inadequate mixing of the combustion air and fuel. One product may be unburned fuel. In severe cases of incomplete combustion, the unburned fuel appears as smoke. Smoke is mostly unburned carbon. The amount of smoke in the flue gas is measured in terms of a parameter called opacity.
Another constituent of flue gas that results from incomplete combustion is carbon monoxide. Carbon monoxide (CO) is produced when insufficient oxygen is supplied to burn carbon completely. When there is good combustion with adequate excess air and mixing, very little CO is produced, so little that very sensitive instruments must be used to detect CO.
When there is significant CO in the flue gas, it is an indication of a serious combustion problem. Modern flue gas analysis instruments can detect very low concentrations of CO. Since CO increases noticeably before the combustion process deteriorates so much that there is smoking, it is a valuable early warning indicator of combustion problems.
Unburned fuel is another possible constituent of flue gas when there is incomplete combustion. Unburned fuel can be dangerous because it makes the flue gas flammable and even explosive.
The undesirables
The undesirable products of combustion are sulfur oxides, NOx, Carbon monoxide, unburned fuel, and ash.
Sulfur Oxides: Sulfur oxides can cause corrosion problems if flue gas temperatures drop below the acid dew point. The acid dew point depends on the amount of sulfur oxides and water in the flue gas. To avoid problems, the flue gas temperature must be kept above the acid dew point. This is TYPICALLY accomplished by controlling the firing rate.
Another major concern is that sulfur oxides are air pollutants. Sulfur oxide emissions are limited by law. Plants burning high sulfur content fuel are often equipped with Flue Gas Desulfurization (FGD) equipment. FGD equipment is very expensive, however, and so, for many plants, the only way to control emissions of sulfur oxides is to burn fuel with very little sulfur content. Typically coal has the greatest amount of sulfur, oil less and natural gas essentially no sulfur. Some fuel oils have relatively high sulfur content while others have very little. As might be expected, low sulfur fuel oil commands a premium price.
Nox: Nox is produced by excessively high temperature combustion. Like sulfur oxides, the amount of NOx that can be released is limited by law. Fuel burners have been developed that limit the amount of NOx produced by causing the fuel to burn at lower temperatures. This is sometimes accomplished by reinjecting flue gas in the combustion process. When burners are properly adjusted, the amount of NOx produced should be within acceptable limits.
Unburned fuel: As described above, unburned fuel results from incomplete combustion. It is dangerous because of the potential for fire and explosion.
Carbon Monoxide: Carbon monoxide is not undesirable in itself, but its presence indicates inadequate excess air or incomplete combustion.