Heat Transfer
Much of the conversion of energy in the steam plant depends upon heat transfer. Heat Transfer is the transmission of heat energy. Heat transfer may be though of as the flow of heat energy from one object (or substance) to another.
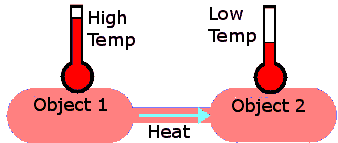
In order for heat transfer to occur, two objects must have different temperatures. Heat energy moves from the high temperature object to the object with the lower temperature. No heat is transferred if the two objects are at the same temperature.
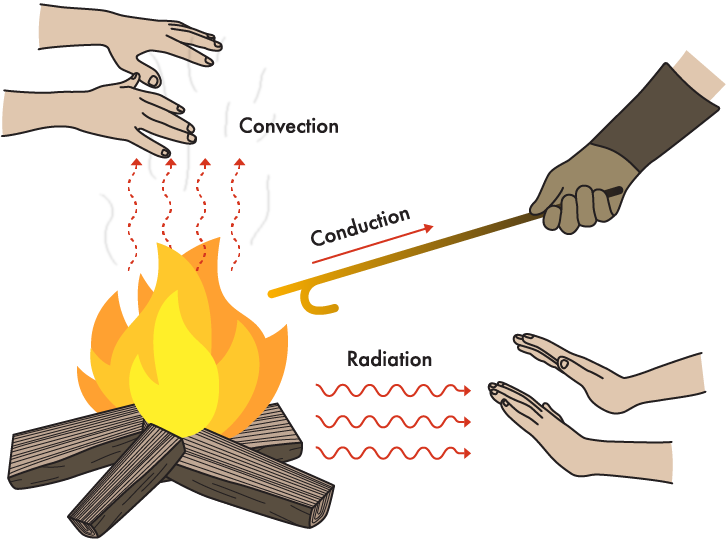
There are three mechanisms of heat transfer, conduction, convection, and radiation. In most situations where there is heat transfer in the plant, all three mechanisms contribute to the heat transfer. We will talk about these below.
Conduction
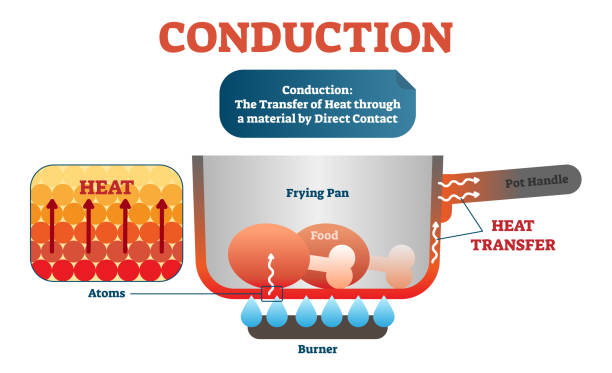
The internal energy of an object (or substance) depends upon molecular motion within the substance. As the temperature of a substance increases, the molecular motion increases, and thus the internal energy increases as the temperature increases. In conduction, heat is transferred from molecule to molecule within a substance or between touching substances by impact of high energy molecules against lower energy molecules.
An example of conduction occurs in a boiler tube. The inside of the tube is kept at a relatively low temperature through convective heat transfer from the water-steam mixture flowing through the tube. The motion of molecules on the outside of the tube (inside the boiler) increases due to heat transfer from all three heat transfer mechanisms. Within the tube wall, heat is conducted from the outside wall to the inside wall. This occurs because the high energy (fast moving) molecules at the outside of the tube impact lower energy (slower moving) molecules inside the tube. The speed of the lower energy molecules is increased by the impact and these “newly energized” molecules in turn impact lower energy molecules and increase their energy. The further from the hot end of the bar the molecules are, the lower their energy and thus temperature.
The amount of heat that is transferred through the boiler tube by conduction depends upon:
- The surface area of the tube (per unit length) – the greater the area, the more heat is transferred
- The thickness of the tube wall – the thinner the wall, the more heat is transferred
- The temperature difference across the tube wall – the greater the temperature difference, the greater the heat transferred
- The conductivity of the material – the higher the conductivity the greater the heat transferred
Given these facts, it would appear that the best boiler tube would be one with a large area, thin tube walls and high thermal conductivity. This tube would be operated with very cold water-steam inside and very hot conditions in the boiler. Practical considerations such as strength require compromises in all of these factors in practice. For instance, the thermal conductivity of copper is higher than that of steel, but the strength of copper at high temperatures is too low to make it a practical boiler tube material. The temperature inside of the furnace is limited by the melting point of the tube material and the temperature inside the tube is determined by the boiler operating pressure (since saturation conditions exists).
Convection
Heat transfer by convection occurs as a result of motion and mixing of a fluid. Convective heat transfer generally occurs between a solid and a fluid (which may be a gas or liquid) flowing over the solid through a combination of molecular motion and fluid motion. Convection is similar to conduction except the fluid is moving, carrying its heat energy with it and so convection involves the physical transfer of heat by fluid motion and mixing. The greater the velocity of the fluid with respect to the solid, the more heat is transferred because greater mixing (turbulence) occurs. The heat energy transfer between individual molecules of fluid, or between the solid surface and the fluid still takes place by conduction however.
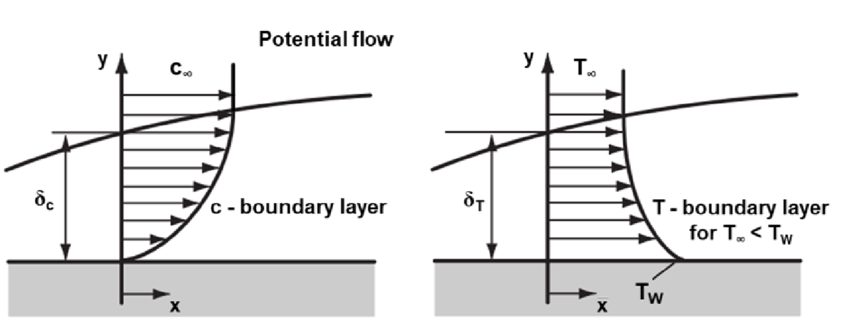
In convective heat transfer, heat is transferred from the solid surface, through a thin layer of fluid at the surface of the solid called the boundary layer to the fluid outside of the boundary layer. The velocity of the fluid molecules on the surface of the solid is zero. The velocity of the fluid molecules increases as the distance from the surface of the solid increases. The boundary layer ends where the velocity of the fluid reaches a constant value compared with that of the fluid outside of the boundary layer.
The boundary layer is very thin, only a few thousandths of an inch, for most convective heat transfer. The reason that it is important is that it can be thought of as a layer of insulation between the surface of the solid and the body of the fluid. The thicker the boundary layer, the less heat transfer takes place. The thickness of the boundary layer, and thus the heat transfer, depends upon the velocity and turbulence of the fluid. The higher the velocity and the greater the turbulence, the thinner the boundary layer.
There are two types of convective heat transfer, free (referred to as natural convection) and forced convection. Natural convection results from fluid motion that is due to local density differences alone. Natural convection is generally low velocity with little turbulence and mixing. Forced convection results when devices such as fans or pumps give motion to the fluids. The heat transfer rates for forced convection are usually greater than those for natural convection since the velocity and turbulence of the flow are usually greater than for natural convection.
Radiation
The third type of heat transfer is radiation. Heat radiation (also called thermal radiation and infra-red radiation) is similar to other types of radiation like light, ultraviolet radiation, and X-rays. All radiation is propagated, like sound, as waves with different frequencies. Different frequencies in sound determine whether the pitch is high or low. Different frequencies in radiation determine whether there is heat radiation or some other type.
Thermal radiation is continuously emitted and absorbed by all objects (substances). The amount of radiation produced by an object is determined by its temperature. Energy at the body’s surface is converted into electromagnetic waves that emanate from the surface and may strike another object. Some of the thermal radiation is absorbed by the receiving object is reconverted into internal energy, causing the received objects temperature to increase. The thermal radiation that is not absorbed is reflected from or transmitted through the object.
Heat transfer by radiation is determined by the temperature of the body radiating heat and the color of the bodies transmitting and receiving the heat energy. The greater the temperature of the body transmitting the heat, the more heat it radiates. The closer the color of the body transmitting to black, the more heat is radiated. Bodies with surfaces having light colors or polished surfaces radiate less heat. Similarly, the heat transfer to a body by radiant heat transfer depends upon its color. Dark colors absorb more radiant heat than light colors. Radiant heat that is not absorbed is reflected.
The amount of radiant heat emitted by an object (substance) is proportional to the temperature to the fourth power. This means that if one were to double the temperature of an object, the amount of radiant heat transfer would increase by a factor of 16. In practice, this means that very little radiant heat transfer occurs at temperatures near room temperature. In general, radiant heat transfer is significant (as compared to conductive and convective heat transfer) only when temperatures increase to the vicinity of 1000°F and above. At these temperatures, considerable visible radiation (light) is also generated. The only place in most plants where such high temperature exists is in the furnace of the boiler. In fact, the boiler furnace is the only location in the plant where radiant heat transfer is significant.