Water and steam
Water is the primary substance used to transfer energy in a steam plant. Much of the transfer of energy comes about through changes of state for the water/steam. Water is used as the working fluid because it is easily available, cheap, is not toxic and has properties that make it well suited for use in boilers and heating systems. In this article the following properties of water are discussed:
- States or Phases
- Heat Capacity (specific Heat)
- Heat of Fusion
- Heat of Vaporization
- Saturation Temperature
- Saturation Pressure
- Superheat
Properties of water
Water can exist as ice (solid), water (liquid), or steam (gas). The state of water depends on its temperature and pressure. At atmospheric pressure, water at 32°F but below 212°F is liquid, and water above 212°F is steam. Heat must be transferred to water to change both its temperature and state.
Consider a piece of ice that is far below the freezing point of 32°F. Heat must be added to the ice to increase its temperature to the freezing point of 32°F. The amount of heat needed to increase the temperature of the ice is determined by its specific heat. Specific heat is basically the ratio of the heat added (or removed) to the temperature change.
The specific heat of ice is 0.505 BTU/lb-°F, and so one pound of ice must absorb 0.505 BUT of heat to raise its temperature by 1°F.
Once the ice reaches the freezing point, however, the addition of more heat does not increase its temperature any more. Additional heat melts the ice instead. The process of melting ice to water is also a change of state. The amount of heat required to melt ice is called the heat of fusion or latent heat. The heat of fusion is the diffirence in internal energy of ice and water. The head of fusion for 1 pound of ice is 144 BTU. If there is one pound of water at exactly 32°F, the same amount of heat must be removed to freeze the entire pound into ice.
When the ice is completly melted, addition of more heat increases the temperature of the water. There is roughly 1.176°F rise for each British Thermal Unit (BTU) added, and so the specific heat of water is about 1 BTU/lb-°F. Actually the specific heat of water changes slightly as its temperature changes and is exactly 1 BTU/lb-°F when the temperature of the water is at 60°F. To increase the temperature of 1 pound of water from 32°F to 212°F, 180 BTU of heat are required. This heat is sometimes called sensible heat, since the head addition can be sensed as the temperature change.
In simpler words:
Latent heat- is the heat added to change the state of the medium with no temperature change.
Sensible heat- noticible temperature change.
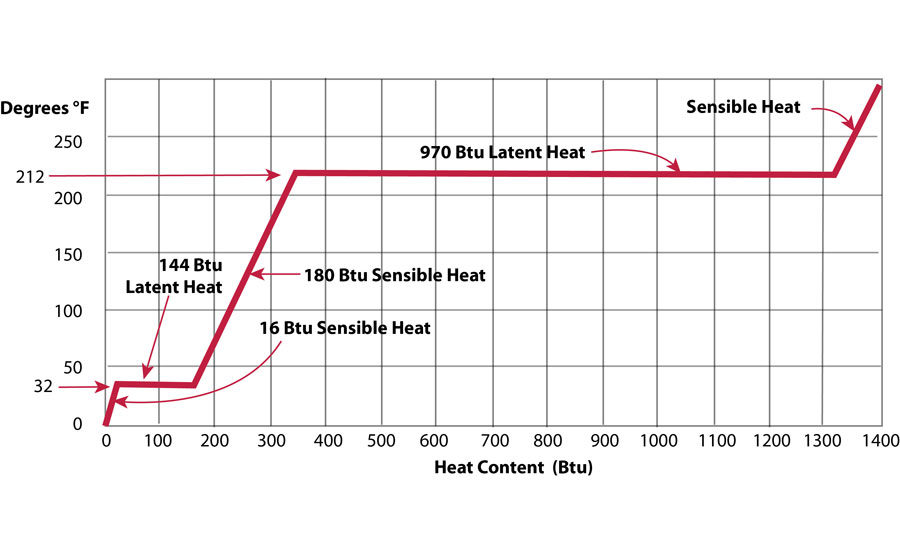
At 212°F another change in state happens. As more heat is added, the temperature of the water does not change. The water starts to boil instead. The reason is that, as with the change of state from ice to water, heat energy, called the heat of vaporization, must be added. The heat of vaporization is the difference in internal energy of water and steam. The amount of heat needed to change 1 pound of water at 212°F to steam at 212°F is 970.3 BTU.
The temperature at which water boils, for a given pressure, is called the saturation temperature. Water at the saturation temperature is called saturated liquid, and steam at the saturation temperature is called saturated steam. At the saturation temperature, water as a liquid and water as a gas exist together. The difference in their heat energy is the heat of vaporization.
The saturation temperature (boiling point) of water depends on its pressure. The saturation temperature of water at atmospheric pressure is 212°F. The saturation temperature of water decreases as its pressure decreases and increases as its pressure increases. The saturation temperature of water at 1 psia (compared to atmospheric pressure of 14.69 psia) is only 101.7°F. The saturation temperature of water at 100 psia is much higher, 327.8°F.
The pressure and temperature of any substance like water are dependent upon one another at saturation conditions. This means that for any given pressure, there is one and only one boiling temperature. This means that if one knows the pressure of saturated steam, one can also determine the temperature. Similarly, if the temperature of saturated steam is known, the pressure can be determined.
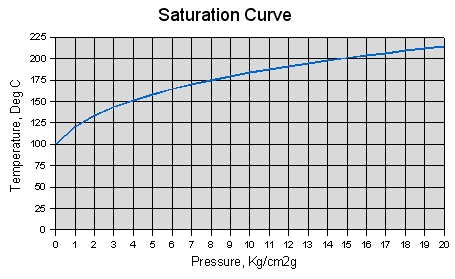
When the heat of vaporization has been added to the water to convert it all to steam, addition of more heat increases the steam temperature above the boiling point. Steam that is above the boling point is said to be superheated. At atmospheric pressure and saturated conditions, the heat capacity of steam is 0.490 BTU/lb-°F. Thus, 0.490 BTU is needed for each degree Fahrenheit above the boiling point. As with water, the heat capacity of steam changes as its pressure and temperature change.
The difference in the temperature of superheated steam and the saturation temperature for its pressure is called the superheat or degrees of superheat of the steam. For example, steam at atmospheric pressure that has been heated to 222°F has 10 degrees of superheat.
In many situations a mixture of steam and water at saturation conditions exists. It is necessary to consider a new parameter to describe this situation. That parameter is quality. The steam quality is the percentage (by mass) of a steam-water mixture that is steam. Thus if 3/4 of a mixture of steam and water were steam, its quality would be 75%.
Steam Tables
The properties of water have been studied more than those of any other substance, which is why we explore this and try to understand these concepts with water first rather than refrigerant. The properties of water that are most useful in thermodynamics of power plants are specific volume, enthalpy, and entropy. Tables have been developed listing the changes of each property with the resultant changes of others. The two tables most used in steam plant work are the saturated steam tables and superheated steam tables. The saturated steam tables provide the properties of steam and water at saturation conditions while the superheated steam tables provide the properties of steam above saturation temperature. Some steam tables also provide the properties of water below saturation temperature. Some steam tables also provide the properties of water below saturation temperature (called sub-cooled water). All of these properties are, together, referred to as steam tables. These tables go as far as even being previously published as stand alone books. We mention them here so that you’re aware of their existance, if you’re interested to learning a bit more you can click here to go to our steam tables article that covers saturated & superheated steam tables to a short extend.
Specific Volume
The specific volume v of steam is the inverse of its density ρ at a given temperature and pressure.
Specific Volume ν = 1 /ρ
Density is the amount of weight a substance has for a unit volume, usually expressed in lb/ft3. Specific volume is, therefore, the volume of a unit mass a substance in ft3/lb. Understanding that the density and specific volume of water change with temperature and pressure is important because some plant equipment takes advantage of this characteristic of water. For example, the steam drum, water tube, and a downcomer shown below use density changes in water for natural circulation.
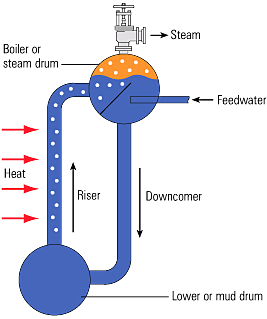
For sakes of an example, lets say in the image above saturated water at 548°F from the steam drum flows through the downcomer. Saturation pressure for this temperature is 1028.49 psia. A saturated steam table can give you specific volume for saturated water at 548°F as 0.02169 x cu ft/lb. The water from the downcomer is distributed to the water wall tubes by the lower header then flows up the water wall tubes located in the walls of the boiler. The water in the tubes absorbs heat from combustion in the boiler. However, since the water is already at saturation temperature, the head added causes some of the water to boil, making saturated steam. From the same saturated steam temperature table, the specific volume for saturated steam at 548°F is 0.43217 x ft3/lb. The ratio of the specific volume of water to steam at this temperature is about 19.9. Another way of expressing this relationship is the water is about 19.9 times more dense than the steam. As a result of this difference in density, the steam bubbles rise in the tubes.
Thus, there is a mixture of steam bubbles and water in the evaporator tubes. There is only water in the downcomer, however, since the mixture of water and steam in the evaporator tubes is less dense than the water in the downcomer, there is greater pressure at the bottom of the downcomer than the bottom of the evaporator tubes. This through the evaporator tubes and back to the drum. This phenomenon is called natural cirulation.