If you’ve been in the industry for some time its likely you’ve heard of the HVAC/Plant operator simplification of thermodynamics, if you’ve been working in steam and power generation its likely you’ve heard of the Rankine Cycle and Brayton Cycle, here we will work our way up to such cycles uncovering the sole basics of how all we essentially do is convert energy. I say overview because there will be articles that unfold these concepts further in specific ways, I do believe you can write a whole article for the refrigeration cycle, for the ranking and brayton cycles, so for the sake of not making this whole article a white paper we will simply discuss the utter basics and give overviews of such items.
Basic Thermodynamics Principles
Thermodynamics is the science that describes and defines the conversion of one form of energy into another. An example is the conversion of chemical energy into thermal energy, which occurs during the combustion process in a boiler for example. For the sake of understanding the basic thermodynamic principles I have personally noticed that understanding it at a steam & water level first really helps when its time to understanding how it comes into play in the refrigeration cycle.
The water and steam used in a boiler plant may be viewed as the working fluid. The working fluid conveys energy between different components. In Power plants the energy in the working fluid is used to drive a turbine-generator to produce electricity. In a heating plant the working fluid is used to heat buildings, and in process plants used to provide the head needed to produce a product. The steam undergoes several changes or processes due to the conversion of energy. This training module will explore this as it relates to energy, work, and heat with the working fluid inside a typical boiler.
Energy
Energy is typically thought of as the ability or capacity to do work. There are many different types of energy, and, in accordance with the law of conservation of energy, it is possible to convert energy from one form to another.
Law Of conservation of energy: This law states that energy can be converted from one form, or type, to another, but it cannot be created or destroyed. This law is known as the first law of thermodynamics. The second law of thermodynamics states that:
Heat always travels from a hot body (high temperature); to a cold one ( low temperature); the greater the temperature difference, the faster the heat transfer will take place.
Two forms of energy are important, in the operation of a steam plant- chemical energy and heat energy and mechanical and electrical are important if the steam produced is used to produce electrical energy.
Chemical Energy
Chemical energy is the energy in the fuel before it is burned. Chemical energy is the energy locked in the molecular bonds of a chemical compound (fuel in this case) with high “energy level.” The chemical energy is released in the process called combustion that occurs when oxygen and heat are supplied in the proper quantities and conditions to burn the fuel. The chemical structure of the fuel is changed when burned and the combustion products (flue gas) that results are at a lower energy level. The difference in the chemical energy level of the fuel and the combustion products is converted to heat energy (enthalpy).
Heat Energy
Heat energy is the energy in a substance that is due to its temperature and pressure. There are two components of heat energy, internal energy and pressure-volume energy.
The internal energy of a substance is determined by its temperature. The motion of molecules of a substance is internal energy. Molecules in a substance are always in motion. The magnitude of the motion in a substance is determined by its temperature. The higher the temperature, the greater the molecular motion and so the more its internal energy.
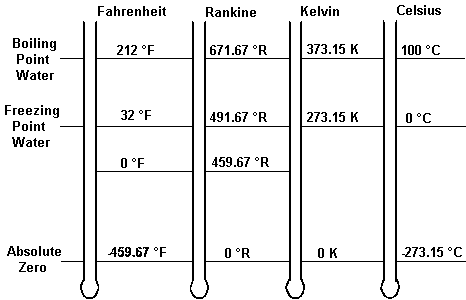
Temperature can be expressed in many different scales, in the english system the fahrenheit scale is defined with the freezing point for water at 32°F and the boiling point (this is all at sea level) is at 212°F. There is another scale that is important in thermodynamics that is significant with regard to internal energy called the rankine scale. The “zero point” for the rankine temperature scale (zero degrees Ranking or 0°R) is “absolute zero.” Absolute zero is the temperature at which, in theory, all molecular motion would stop. This temperature is very low; -459.68°F. The internal energy of any substance at absolute zero would be zero since internal energy is determined by molecular motion. The Rankine temperature scale must be used in areas of thermodynamics by engineers involved in the design of plants. For specific conversions and information about that click here>>>>>.
Fahrenheit and Rankine are English units. In the metric system that is used by most of the world, temperature is measured in the Celsius and Kelvin scale with Kelvin being the units for absolute temperature.
The amount of internal energy a substance has depends upon the substance as well as its temperature. THis can be seen in the amount of energy it takes to change the temperature of different substances. To increase the temperature of 1 pound of water by 1 degree Fahrenheit (°F) at 60°F, it takes 1 british Thermal Unit (BTU). Only 0.118 BTU is required to change the temperature of 1 pount of steel at the same temperature by 1°F, therefore one can say that 1 pound of water has more internal energy than 1 pound of steel.
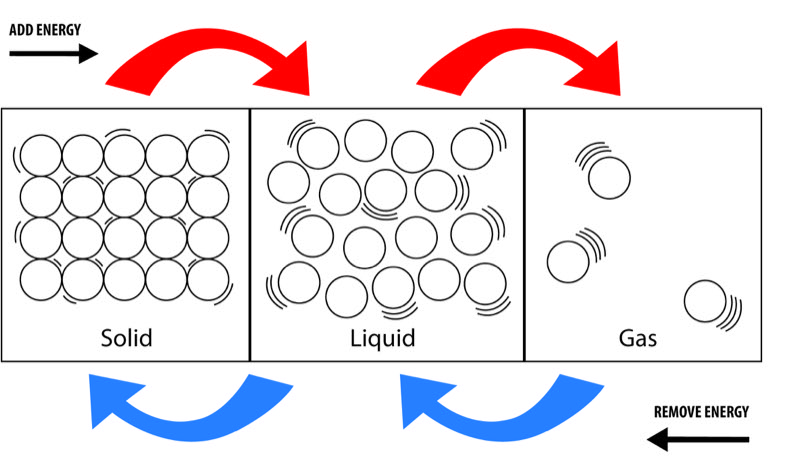
The amount of heat energy in a substance also depends on its state; solid, liquid, or gas. Some of this difference is usually because there is a difference in temperature of a substance in these states (solid coldest, gas hottest). Even when the substances are the same temperature (ice in water for instance(, there is a significant difference in the heat energy between a substance in different states. This example of ice water, the heat energy between a substance in different states. In the example of ice in water, water at the freezing point has much more heat energy than ice at the freezing point. This difference in energy occurs because as heat energy is added and the substance goes from solid to liquid and then from liquid to gas, the molecular structure becomes more random and there is more molecular motion. Thus, steam at the boiling temperature has more heat energy than water at the same temperature because molecules of steam are in greater motion than those of a liquid.
Gas molecules are at a higher energy level than liquid or solids and are free to seperate and move. Because of this fact, gases like air or steam are compressible. This means that if the pressure of a given volume of gas is increased, its volume decreases significantly. For example, if there is a cubic foot of air in a cyclinder with a piston at atmospheric pressure (about 14.7 psia) the air can be compressed to half its volume (1/2 cubic foot) by pushing on the piston and increasing the air pressure to two atmospheres (about 29.4 psia). Energy must be expended in pushing the piston to compress the air, however, and this energy appears in the air. The energy in the air is often called pressure-volume energy since it depends on the pressure and volume of the gas.
Pressure in the English system is measured in pounds per square inch. There are two variations in pressure. The first of these, and the most common is pounds per square inch gauge (psig). Atmospheric pressure is defined as zero psig. The other variation is pounds per square inch absolute (psia). A perfect vacuum is defined as zero psia. Atmospheric pressure at sea level is 14.69 psia. The absolute scale is often used in thermodynamics.
It should be apparant from this discussion, then, that the amount of energy in a substance like steam depends upon both its temperature and pressure. In addition, the state of the substance, solid, liquid or gas, also has considerable influence on the energy. Click here for more information on conversions or equations as they portrain to this, you can see a deeper level of how the heat energy or enthalpy of a substance is the sum of its internal energy and its pressure-volume energy.
Mechanical Energy
Mechanical energy is made up of two different components, potential energy and kinetic enregy. Potential energy is the energy a body has as a result of its distance from the center of the earth, or its elevation. The higher the elevation of a substance the more potential energy the body has.
Kinetic energy is the energy that a substance has a result of its velocity. The higher the velocity of a substance, the more kinetic energy it has. The kinetic energy in a substance is proportional to the square of its velocity. Thus, if one were to double the velocity of an object like a ball, its kinetic energy would quadruple.
A body, such as a ball, generally has both potential and kinetic energy. This is true for instance for a ball that has been thrown into the air and is 20 feet above the ground and has a velocity of 40 feet per second. The sum of potential and kinetic energy of the ball is its mechanical energy. While there are changes in both potential and kinetic energy in the power plant cycle, the role potential energy plays in the overall energy conversion is relatively unimportant as compared to the other forms of energy used in the power plant cycle.
Electrical Energy
Electrical energy results from electrons flowing through a conductor such as copper wire. The amount of electrical energy flowing through a conductor is determined by the electron flow, what may be considered as the “electrical pressure” against which the electrons must flow, and the duration of that flow. The greater the electron flow, measured in amps, the greater the electrical energy. The greater the “electrical pressure,” measured in volts, the greater the electrical energy. Electrical energy is usually expressed in terms of watt-hours which are the product of volts (E), amps (I), and time.
Units of Energy and Work
Units are used to describe the size and magnitude of various properties of matter. Many properties of matter, including temperature, have different units in different measurement systems. In the discussion of temperature above, for example, it was explained that the unit degree Fahrenheit can be used to express the temperature of a substance. There are many other temperatur escales such as Centrigrade and Kelvin that are not addressed in this text.
Work, energy and other properties of substance are expressed in many different units as well. The choice of units often depends on the disicipline being considered. When working with electrical equipment, it is convenient to use electrical units such as volts, amps and watts. When Working with mechanical components, it is convenient to work in mechanical units such as pounds, feet, inches, foot-pounds, and BTU’s.
Since the same paramater may be expressed in different units, it is often necessary to “convert” the units. This conversion from one property to another is done by using conversion factors and formulas. An example of a conversion factor has already been given in the discussion on temperature scales above where you can access it by clicking the link. Temperature can be converted from degree Fahrenheit to degrees Rankine by adding the conversion factor 459.67. The formula for conversion in this case is simply:
In many cases conversion factors must be used by multiplying or dividing rather than adding or subtracting. Conversion factors are published in many different places including many books that have steam tables.
Common examples of prefixes commonly used with units are “kilo” which means one thousand, and “mega” which means million. A conversion factor is implied when these prefixes are used. For example one kilowatt is equal to 1,000 watts. The conersion factor in this instance is 1000 watts per kilowatt.
Heat energy = Btu
Mech energy = Ft-lbs
Elec Energy = Watts
1 KWH = 3413 BTU
1 BTU = 778 ft-lb
1 HP = 550 ft-lb/sec
1 atmosphere = 14.69 psia
1 atmosphere = 29.921 in HgA
1 psia = 2.037 in HgA
It is also common to use abbreviations with units. Examples of common abbreviations are “°F” for degrees Fahrenheit, “KW” for kolowatts and “BTU” for british Thermal Unit. Conversion tables usually provide these abbreviations as well as the conversion factors.
Conversion factors are used in the following example in which the efficiency of a power plant is determined. A power plant burns coal that has a heating value of 11,500 BTU/lb at a rate of 550,000 pounds per hour and produces 680,000 KW of electricity. The power plant produces 6.32 billion BTU per hour through conversion of chemical to heat energy by burning the coal. The power plant also produces 680,000 KW-hours (KWH) of electrical energy per hour. The efficiency of power plants in the United States is commonly expressed in units of BTU/KWH. One could express BTU/KWH as “how many BTU’s must be expressed in units of BTU/KWH. One could express BTU/KWH as “how many BTU’s must be added to the cycle to produce a KWH of electrical energy.” In this case the answer is 9,301 BTU/KWH. It is also possible to use a ratio of the output to the input to determine the efficiency on a percentage basis. Using the conversion 1 KWH = 3413 BTU in the equiation below, this power plant is 36.7% efficient, that is 36.7% of the energy in the coal received by the plant is converted to useful electrical power.
Work
Energy is defined as the ability to do work. One way to define work is in terms of mechanical energy. In these terms, work is the action of a force through a distance, usually in moving an object. In fact, work is often considered as “energy in motion.” As mentioned earlier, the units of work are foot-pounds. If a person pushes a block of metal 10 feet with a force of one pound, then 10 ft-lb of work is done on the block when it is moved 1 foot.
Work is also considered to be mechanical energy since moving an object increase its kinetic and/or potential energy. Work can be though of as a way to convert one type of energy to another. Thus, if a man lifts a 50 pound weight from the floor to the top of a table that is three feet high he does work on the weight by exerting a force of 50 lb through a distance of 3 feet, for a total of 150 ft-lb of work. The potential energy of the weight increased by the same amount, 150 ft-lb.
The idea of a force acting through a distance can be extended to rotating equipment like turbines, pumps and fans. In each case, work is done on or by the component through a rotating shaft. That work can be though of as a torque acting through circular motion or rotation of the shaft. The turbine, for example does work on the generator by exerting the force (torque) on the generator as it moves (rotates). The generator then converts the mechanical energy from this work to electrical energy.
Power
It is useful to know how much energy is necessary to make a process occur. The amount of energy alone is not enough to describe many processes, however. The rate at which the energy is delievered to or generated from a process is also important. Power is the rate at which work is done. For example, if a car climbs a hill that is 1000 feet high at 30 miles per hour, a certain amount of work is done on the car by the car’s motor and that work appears as potential energy when the car is at the top of the hill. If the same car travels to the top of the same hill at 60 miles per hour, the same energy conversion takes place, but in half the time. Thus, for the car that climbs the hill at 60 mph the work is done in half the time as the 30 mph car and thus used twice the power.
In duscssing power plant efficiency, the term power is not always used correctly, there is often confusion between power and energy. To understand this statement, consider a generator that operates at powe rof 700 MW. This means that the rate of energy production is 700 MW and could be expressed as 700 MWH (mhegawatt-hours) per hour. The watt-hour is the unit of energy for electricity. A utility is paid based not on the rate at which they generate and deliver electrical energy: rather it is paid for the electrical energy delivered, usually expressed in units of kilowatt-hours (KWH). Indeed, an examination of a household electrical bill will quickly confirm that the amount to be paid depends on the KWH used, not the rate at which it was used.